[…]
[…]
Type II collagen is abundant in the joint and is a major target of immune-mediated damage in RA. As suggested in studies of experimental animals, induction of immune tolerance at the level of the cartilage may be a way of reducing the signs and symptoms of RA and prevent joint destruction. However, whether oral administration of Type II collagen is an efficacious treatment for humans with established active RA has not been established.
Increasing the dose of infliximab and, in some cases, adalimumab can result in improved efficacy in RA patients with inadequate responses to initial dosing. However, whether increasing the dose of etanercept can also result in clinical improvement has not been studied.
Bone erosions in response to inflammation in RA are driven by osteoclasts which are in turn activated by RANKL (Receptor Activator for Nuclear Factor κ B Ligand) binding to its receptor RANK. Inhibition of RANKL has the potential to retard bone erosions in RA patients, thereby limiting progressive joint damage and destruction.
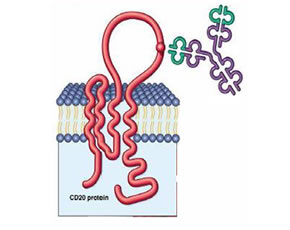
Arthritis News : Golimumab Shows Promise for the Treatment of Rheumatoid Arthritis