Lyme disease is a multi-system bacterial infection caused by the spirochete, Borrelia burgdorferi, which is transmitted to humans primarily through the bite of an infected deer tick. Lyme disease has several different stages: first stage (acute/early localized), second stage (early disseminated), and third stage (chronic/late disseminated). Symptoms vary depending on the stage of illness. In early Lyme disease, patients can present with a rash and/or flu-like symptoms. In later stages symptoms can manifest in multiple body systems, including musculoskeletal, heart, brain, and nervous system.
How has Lyme disease expanded since its first discovery?
Lyme disease, was first discovered in the United States in Lyme, Connecticut, in the mid-1970s and is now the most common US vector-borne disease. The cumulative number of Americans struggling with chronic symptoms relating to Lyme disease is estimated to be in the millions.
The number of Americans diagnosed and treated for Lyme disease in the US exceeds 476,000 per year.
Ticks that transmit Lyme disease are spreading geographically and are found in half of US counties.
Lyme disease is spreading to more locations in the US and is hyper-endemic in the northeast and mid-Atlantic, upper Great Lakes region, and west coast. A worldwide health epidemic, Lyme disease is also a growing problem in regions such as Canada, Europe, and Asia.
Reported Cases of Lyme Disease – United States, 2019:
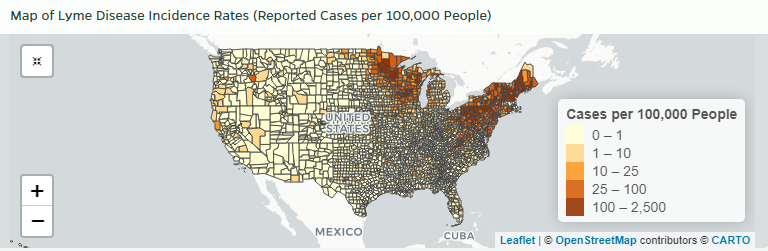
Source: HopkinsLymeTracker.org
Reported Cases of Lyme Disease – United States, 2018:
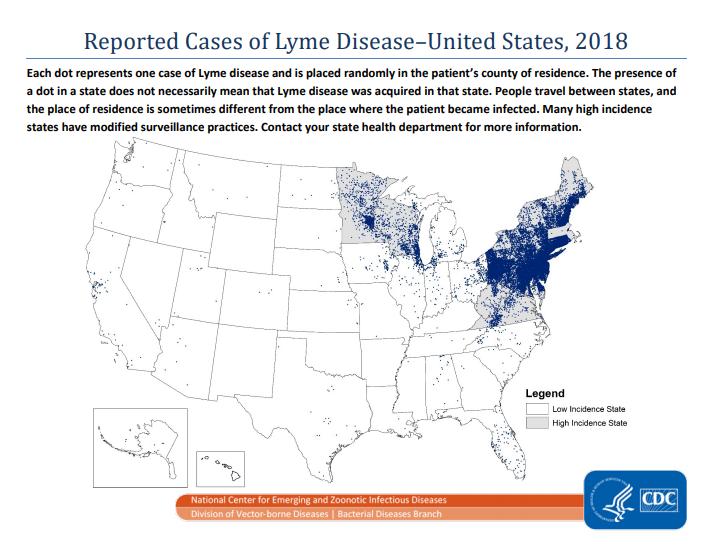
Note that Massachusetts, an endemic state, is no longer reporting Lyme Disease cases
Source: CDC
Reported Cases of Lyme Disease – United States, 2001:
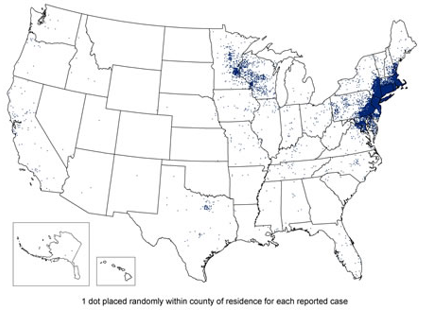
Source: CDC
Reported Distribution of Borrelia Burgdorferi in Blacklegged Ticks – United States, 2021:
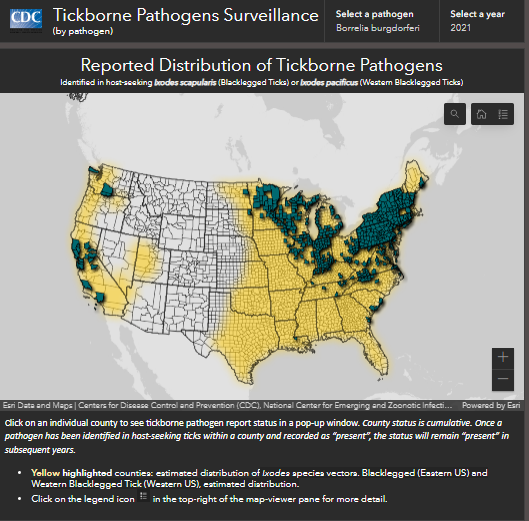
Source: CDC
What causes Lyme Disease?
Lyme disease is primarily transmitted by the bite of an infected black-legged tick, otherwise known as a deer tick. The infective agent of Lyme disease, the spirochete bacteria, Borrelia burgdorferi, is an obligate parasite of animals and requires animal reservoirs, such as mice, to survive. Ticks transmit Borrelia burgdorferi between animals including mice, small rodents, birds, some reptiles, pets, and people. There are numerous genotypes of Borrelia burgdorferi that are associated with different degrees of virulence.
Black-legged ticks can also transmit other infectious agents such as Anaplasma, Babesia, and Powassan virus. In addition, there are other species of borrelia that cause Lyme-like illnesses including Borrelia mayonii, and the relapsing fever like organism, Borrelia miyamotoi. Other tick-borne illnesses include Ehrlichiosis, Rocky Mountain Spotted Fever (& other Rickettsias), and Southern Tick-Associated Rash Illness (STARI). Another condition is alpha-gal meat allergy that occurs in some individuals after being bitten by a lone-star tick.
Who is at risk of Lyme disease?
Lyme disease can affect anyone. People who spend time outdoors, especially in grassy, shrubby, and wooded environments, are at increased risk of exposure particularly in regions where ticks are prevalent and during times of year when ticks are most active.
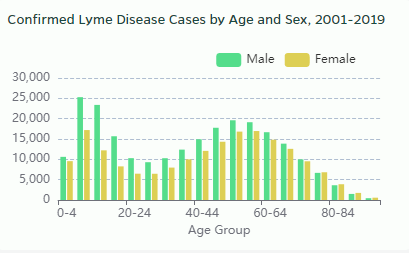
Infection is most common among children aged 5-15 years and adults aged 40-65 years. Boys aged 5-10 are at the greatest risk.
Can tick-borne disease be life-threatening?
Some tick-borne conditions can be serious or life-threatening and may warrant immediate medical attention. If Lyme carditis, Lyme meningitis, Powassan virus, Rocky Mountain spotted fever, Ehrlichiosis, or alpha-gal allergy are suspected, please seek immediate medical attention.


